Computer System Validation Training
CSV Training, a subsidiary of Validation Associates LLC, specializes in software validation, Cleaning validation, Instrument/Equipment Validation, Process Validation, Documentum, Argus, Trackwise, ERP, LIMS, 21 CFR Part 11, training and database development and population. CSV Training is a one-stop shop for all your software validation and database application development needs.
Since 2003 CSV Training has been providing high quality documentation, training, and consulting services in support of the installation and validation of custom and commercial Laboratory Information Management Systems (LIMS), Computerized Laboratory Applications, and Quality Systems. Our Training Courses are designed and developed by compliance professionals for compliance professionals.

Computerised System Validation Overview and Approach
CSV is a practice that ensures the reliability of computer systems used for tasks such as data management, quality control, and regulatory reporting. CSV is vital for the pharmaceutical industry, as a failure can have severe consequences, which can affect patient safety, product quality, and regulatory compliance.
Computer system validation training courses prepare trainees with the skills and knowledge necessary to follow computer system validation procedures to maintain compliance and improve operations. This training course introduces participants to regulatory requirements for computerized systems in the pharmaceutical industry and tried, tested, and internationally recognized methods of meeting those requirements using GAMP 5 guidance.
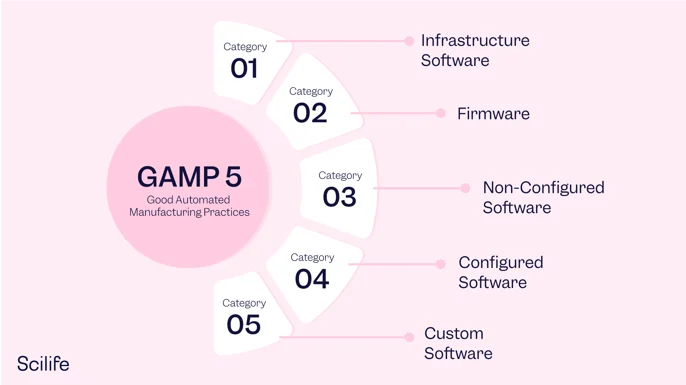
GAMP5 Approach and Documentation
This course describes how the GAMP® Good Practice Guides may be applied to achieve compliance for the systems that are fit for intended use and meet current regulatory requirements. GAMP stands for Good Automated Manufacturing Practice. Generally, GAMP5 refers to a guidance document entitled GAMP®5: A Risk-Based Approach to Compliant GxP Computerized Systems. This document is published by an industry trade group called the International Society of Pharmaceutical Engineering (ISPE) based on input from pharmaceutical industry professionals.
Simply put, GAMP5: A Risk-Based Approach for Compliant GxP Computerized Systems provides a framework for a risk-based approach to computer system validation where a system is evaluated and assigned to a predefined category based on its intended use and complexity. The system categorization helps guide the drafting of system documentation.

Achieving and Maintaining 21 CFR Part 11 Compliance
This course describes definitions and basics of 21 CFR Part 11 rule of US FDA. The program reviews the requirements, FDA guidance and Annex 11 ER/ES expectations from the industry. 21 CFR Part 11 addresses the use of technology in quality systems. Life science companies that implement electronic records or electronic signatures must adhere to 21 CFR Part 11.
21 CFR Part 11 is comprised of three parts. The General Provisions section serves as an introduction. It addresses Part 11’s scope and implementation and provides key definitions pertaining to 21 CFR. The Electronic Records section details the procedures and controls applicable to electronic records. These requirements apply to anyone who uses electronic systems to create, modify, maintain or transmit electronic records and aim to ensure the records’ authenticity, integrity and confidentiality as applicable.
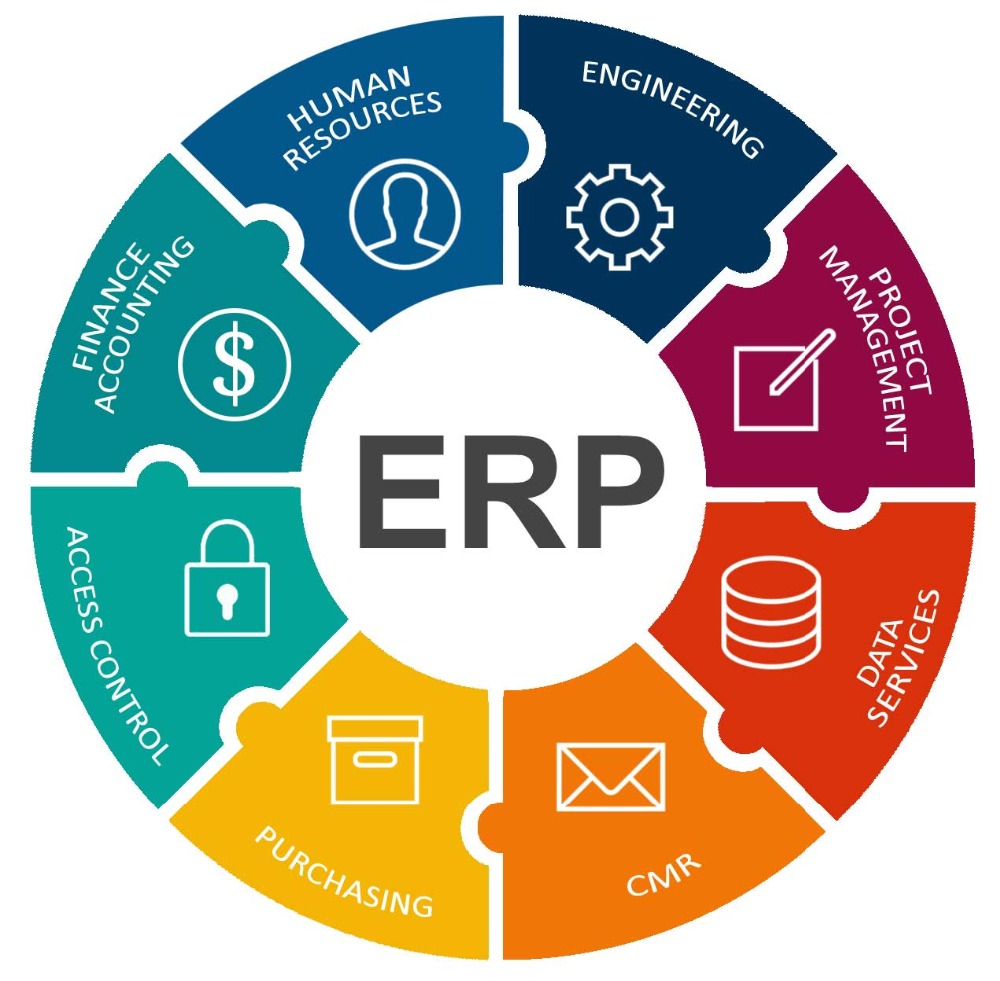
Training-cum-workshop Programme on ERP System (SAP) Validation.
The increased use of information technology and ERP systems in all aspects of manufacturing is leading to the automation of more and more processes. Key decisions and action are routinely being taken using ERP system with regulated records being generated electronically. This course explains basics of Global Information System validation based on GAMP5 practices and documentation that is needed, including electronic records management.
Software validation is required by the FDA and other key regulators to ensure that the pharmaceutical, biotech, and medical device firms' chosen enterprise software systems are fit for purpose and capable of delivering the required outcomes. Regulatory bodies place these responsibilities on companies to improve product quality and consistency and increase patient safety.
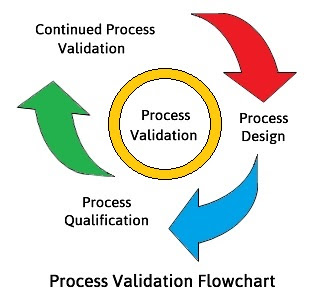
Training-cum-workshop Programme on Process Control System Validation
With the help of this training program, executives will be able to identify correct practices and documentation following GAMP 5 approach for managing such documentation. This will help to reduce repeat documentation, additional time and costs.
Process control systems for pharmaceutical production plants have to be designed, installed and commissioned in accordance with specific quality assurance measures to ensure high operational safety and compliance with regulatory requirements
