Qualification Part
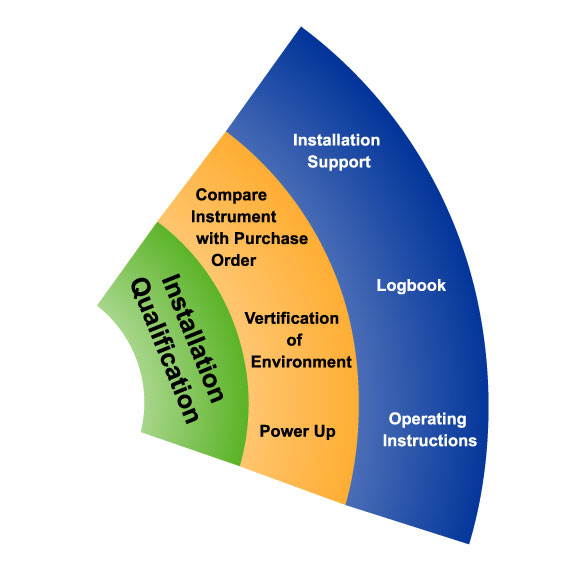
Installation Qualification(IQ)
Installation qualification is used to ensure that the installation of any necessary equipment, piping, services, or instrumentation has been executed in accordance with the manufacturer's requirements.
Before you can test whether your equipment performs correctly, you need to know that it has been delivered, installed, and configured correctly. Installation qualification is the documented process that verifies equipment and any parts that comes with it against a checklist of specifications from the manufacturer.
Operational Qualification(OQ)
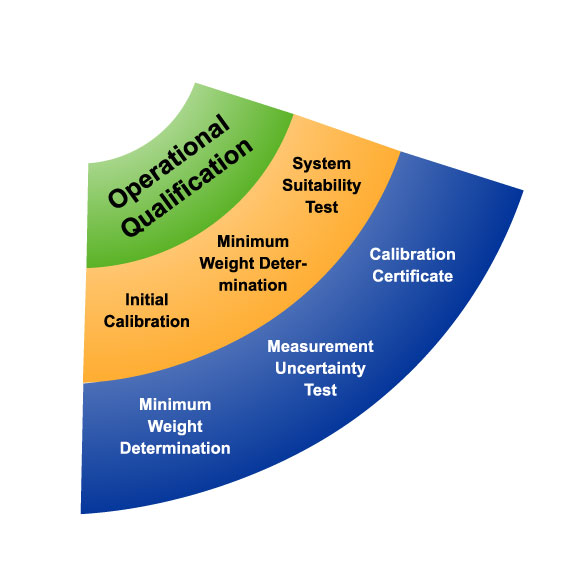
Once the IQ has been conducted, the next stage in process validation—operational qualification—ensures that the equipment is operating in accordance with the user’s requirements and within the operating range specified by the device manufacturer.
During operational qualification, the equipment should be tested to determine process control limits, potential failure modes, action levels, and worst case scenarios. OQ ensures that equipment and systems operate according to their intended design and specifications. It involves testing the equipment under various operational conditions.
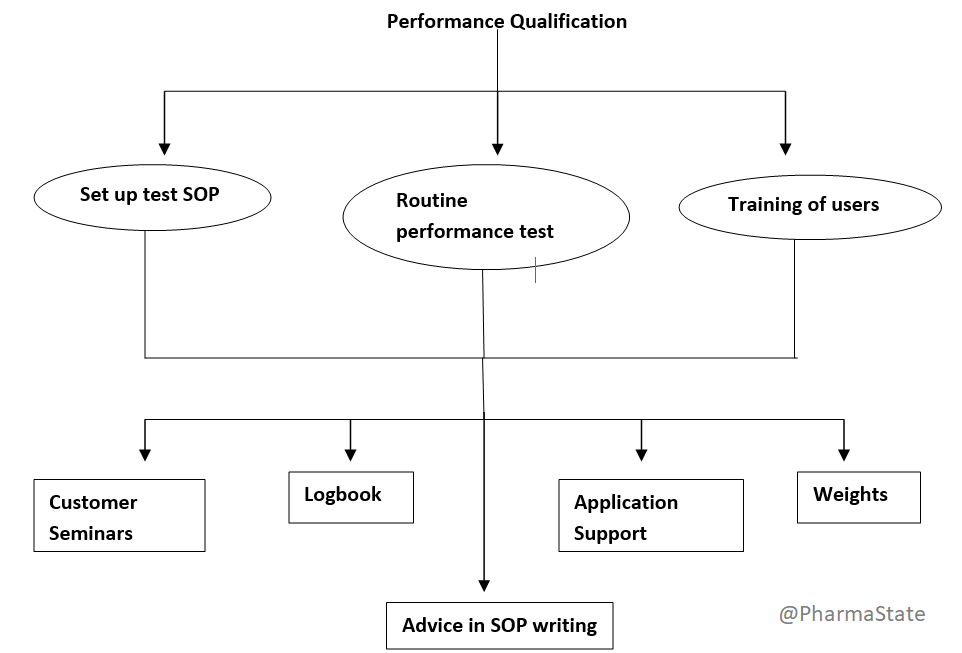
Performance Qualification(PQ)
In the performance qualification phase, the goal is to demonstrate that the process will consistently produce acceptable results under normal operating conditions.
While similar to operational qualification, performance qualification is used to verify that the equipment consistently produces the correct results under real-world conditions. That means PQ should be conducted in the actual facility with trained personnel, using the utilities, equipment, control procedures and manufacturing process that will be used to produce commercial batches of the product.
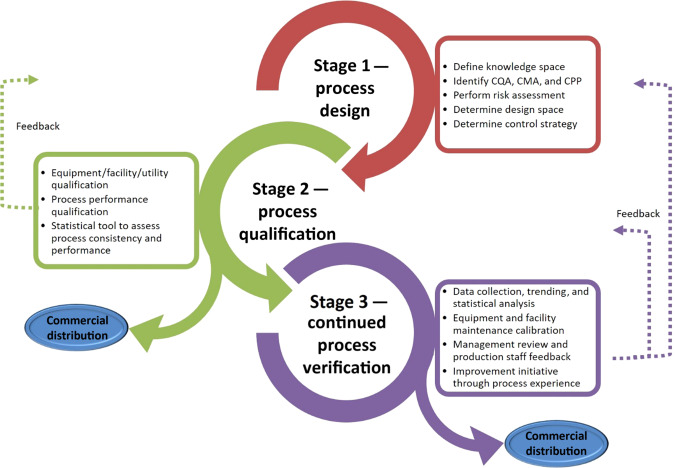
Process Qualification(P2Q)
Process qualification is the qualification of manufacturing and production processes to confirm they are able to operate at a certain standard during sustained commercial manufacturing. Data covering critical process parameters must be recorded and analyzed to ensure critical quality attributes can be guaranteed throughout production
Equally important as qualifying processes and equipment is qualifying software and personnel.[4] A well trained staff and accurate, thorough records helps ensure ongoing protection from process faults and quick recovery from otherwise costly process malfunctions. In many countries qualification measures are also required, especially in the pharmaceutical manufacturing field.
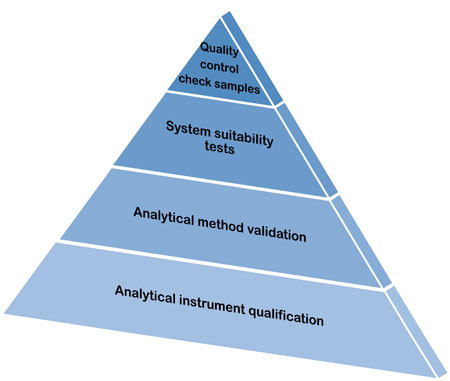
Analytical Method Validation(AMV)
This qualification ensures that analytical methods used to test pharmaceutical products are accurate, reliable, and suitable for their intended purpose. It involves validating methods for parameters such as accuracy, precision, specificity, and sensitivity.
The Objective of Validation of an Analytical procedure is to demonstrate that it is suitable for its intended purpose. It is applicable to identification, Control of Impurities and Assay. Analytical Test Method Validation for Dissolution Method follows almost the same procedure but only differs in the Acceptance criteria.