What is Pharmaceutical Quality Management System (QMS)?
Pharmaceutical Quality Management System (QMS) is a comprehensive collection of policies, processes, and procedures designed to ensure and maintain uniform and high quality in the production of pharmaceutical products.
A robust Pharmaceutical Quality System (PQS) that reflects the applicable requirements can also help pharmaceutical companies to mitigate risks, improve customer satisfaction, and streamline quality processes.
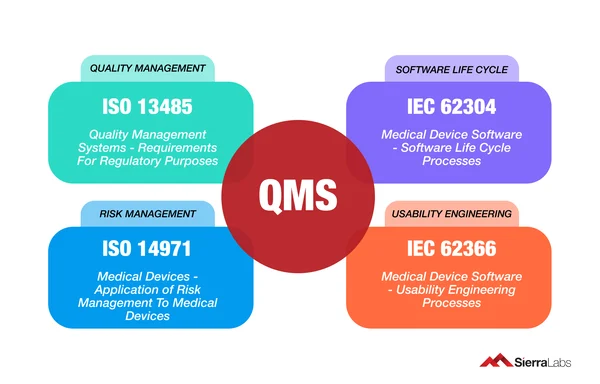
QMS Regulations and Standards
Establishing and working according to the Quality Management System (QMS) is a requirement in the pharmaceutical industry. To ensure uniform and high-quality products, companies need to implement a QMS that reflects the requirements applicable to them.
The company is responsible for complying with the relevant regulations and standards, which depend on many factors, including geographical location, product type, and target market.
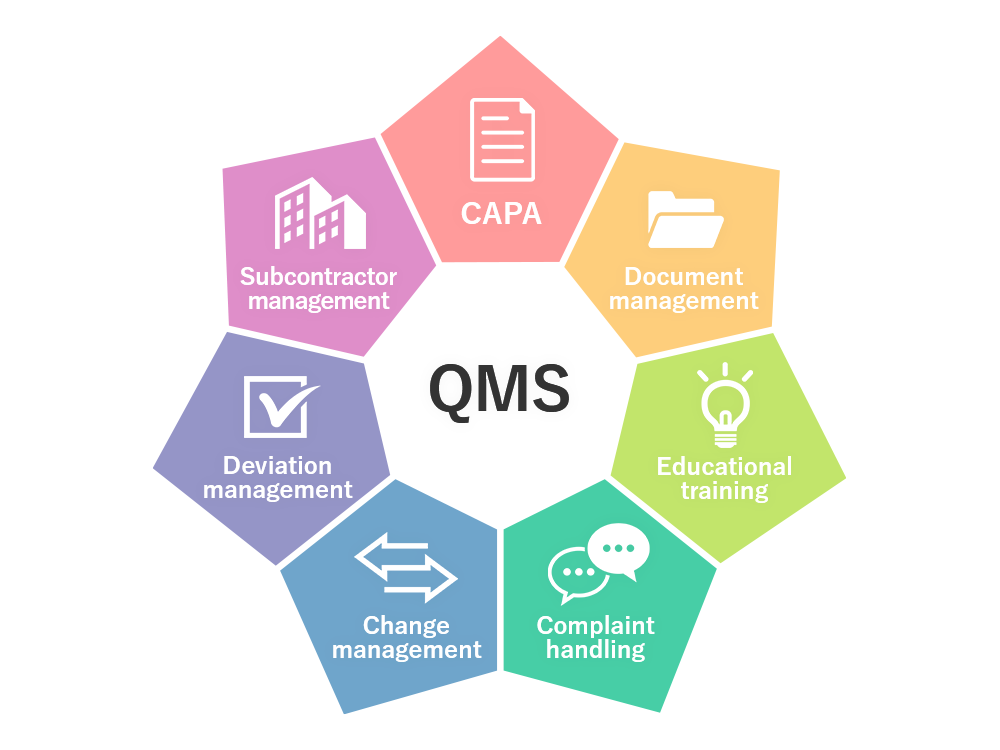
Elements of Pharmaceutical Quality Management System
A Pharmaceutical Quality System comprises numerous elements and QMS processes. There is no “fixed” implementation scheme. The drug manufacturer can integrate these processes as per the product and intended market requirements.
This article will also provide examples of how QMS software can simplify and optimize processes. The process performance and product monitoring system is an integral part of a company’s efforts to deliver a uniform and high-quality product.

Role of Pharmaceutical Quality Management Software
Companies can manage their QMS using manual solutions if the required resources to handle the manual paperwork are available. However, pharmaceutical quality systems can be complex due to the multiple interlinked processes involved and the high level of documentation required.
The digital revolution in drug manufacturing industries has paved the way for more production with less cost and time. Digital tools can take care of routine processes without compromising product quality, allowing company owners to focus more on innovation and exploring new avenues.
Final Thoughts
The applicable regulatory requirements may vary depending on product type and intended market. Some pharmaceutical requirements include the ISO 9001:2015, ICH Q10, EU and PIC/S GMP, FDA 21 CFR Part 210 and 211.
One of the requirements is to implement a pharmaceutical quality management system and adhere to the processes outlined in it. The QMS enables companies to streamline their quality processes and helps ensure compliance to manufacture safe, efficient, and reliable products.

