Course Overview
This Pharma Quality Management Systems course explains how to establish, manage, monitor and continually improve a forward thinking modern Pharmaceutical Quality Management System that adds real-value to your organisation.
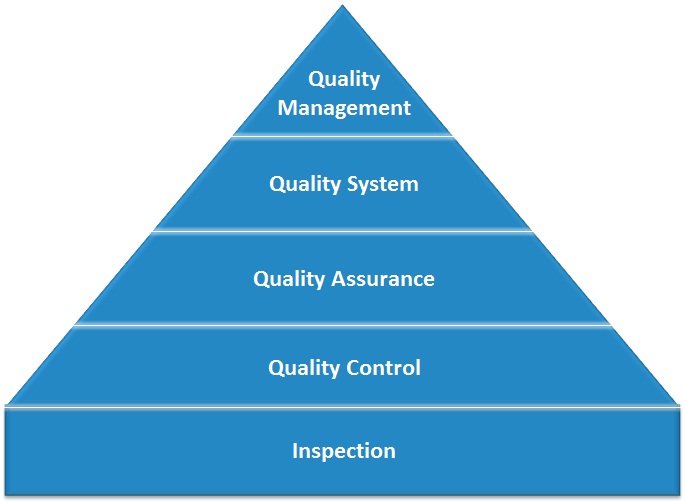
The course covers current principles on Pharmaceutical Quality Management Systems (PQMS) and includes the FDA’s “Guidance for Quality Systems” and ICH Q10 “Guidance on Pharmaceutical Quality Systems”.
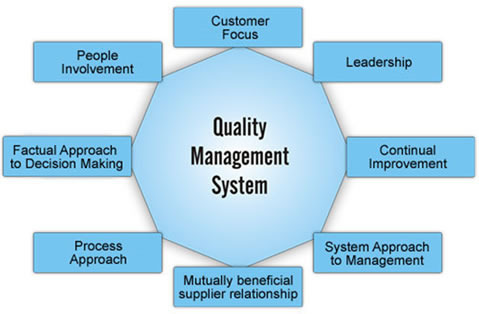
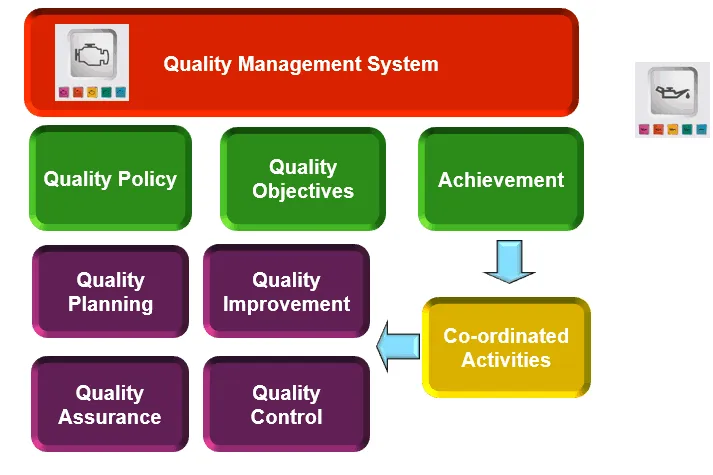
Course benefit
This Pharma Quality Management Systems course is aimed at busy professionals working in the Pharma industry who need to understand how a forward thinking modern Pharmaceutical Quality Management System can add real-value to their organisation.
It is especially valuable for Quality Professionals, especially those working in Quality Assurance and Quality Improvement roles, as the course provides an eye-opening view of modern quality management thinking.
Course contents
The Evolution of Quality Management Systems
- Introduction to the course
- The concept of Quality and Quality Definitions
- The concept of Quality Management System thinking
- The evolution of Quality Management System thinking
- Good Manufacturing Practice – EU and USA GMP
- Pharmaceutical principles of QA, GMP and QC
- Traditional GMP and modern QMS thinking
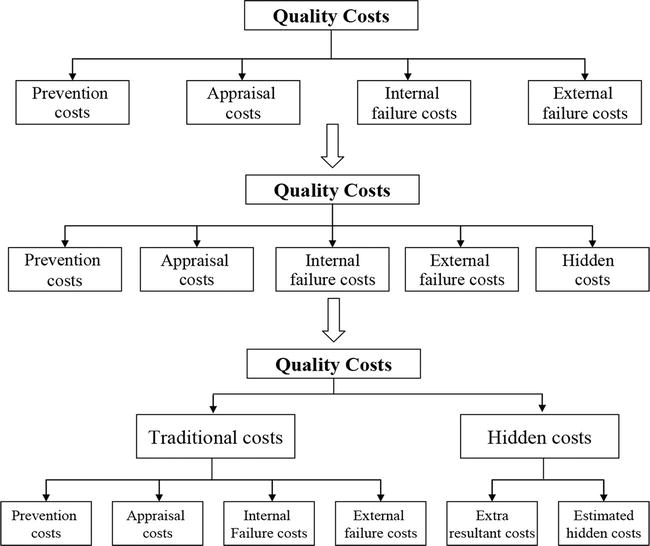
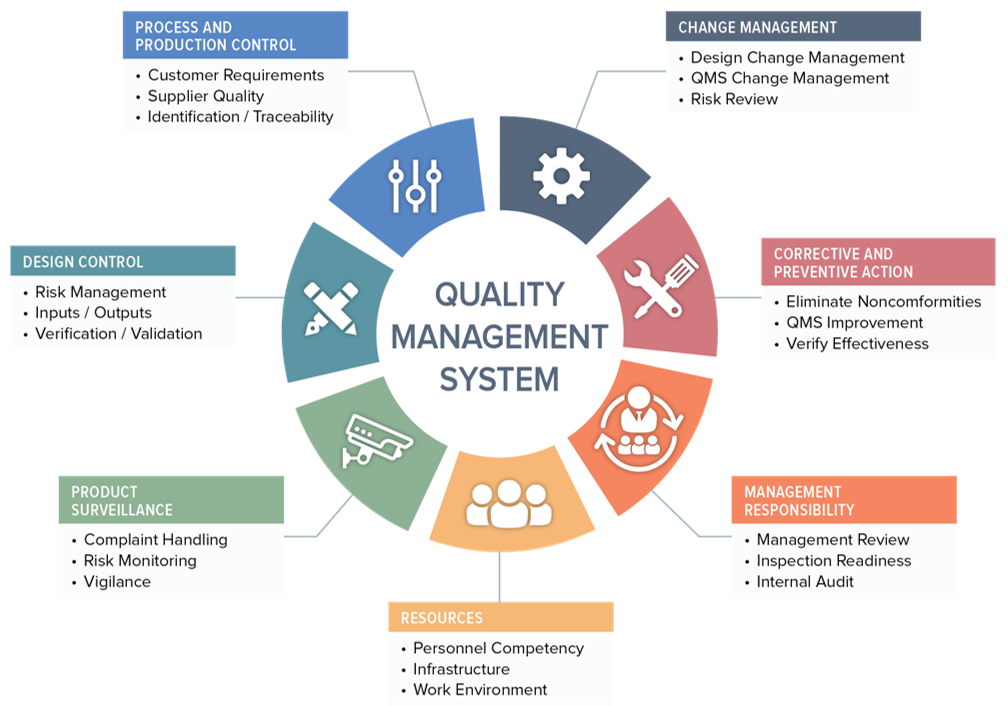
How to Design a Quality Management System
- The design criteria for an effective QMS
- Comparison of ISO 9001 and GMP
- Pharmaceutical QMS evolution
- The FDA’s Quality System Model & ICH Q8, 9 & 10
- Documentation systems, documents and record keeping
- Organisational structures, reporting relationships and review
- The design, selection and qualification of premises, equipment, utilities and services
- The concepts associated with risk management
- Additional guidance for those starting a new QMS
How to Implement a Quality Management System
- Assigning roles and responsibilities
- The roles of the Heads of Production & QC and the Qualified Person
- The role of QA and their interaction with other departments
- The roles and responsibilities of Top Management
- Training and evaluation
- The skills and competencies needed to provide effective GMP training

How to Maintain a Quality Management System
- Review of the system so far
- Purchasing and supplier qualification
- Supply chain, materials control, brokers, distributors and repackagers
- Outsourcing and technical agreements
- Training and evaluation
- Production planning, scheduling and inventory control
- Deviations and change control
- Calibration and preventive maintenance
How to Evaluate a Quality Management System
- Annual Product Quality Reviews
- Economic and statistical indicators
- Staff Appraisals and Performance Management
- Management ReviewAuditing and self-inspection
- The collection and analysis of data


How to Improve a Quality Management System
- Customer complaints and satisfaction monitoring
- Correction, Corrective Action and Preventive Action
- Quality metrics – GMP and beyond
- The Process Approach
- Process based auditing
- Internal customer focus
- Interpersonal skills and required behaviours
