SAP System Validation
The SAP system setup for Pharmaceutical companies is same as any other industries/organizations; just Pharma companies are regulated by special Government bodies by the state.
This is because Pharma companies manufacture medicines and other products which are essential for maintaining good human life and hence are sensitive entities. To launch any new pharmaceutical product in the market, it has to be rigorously tested and test results documented and are verified by the regulating bodies.
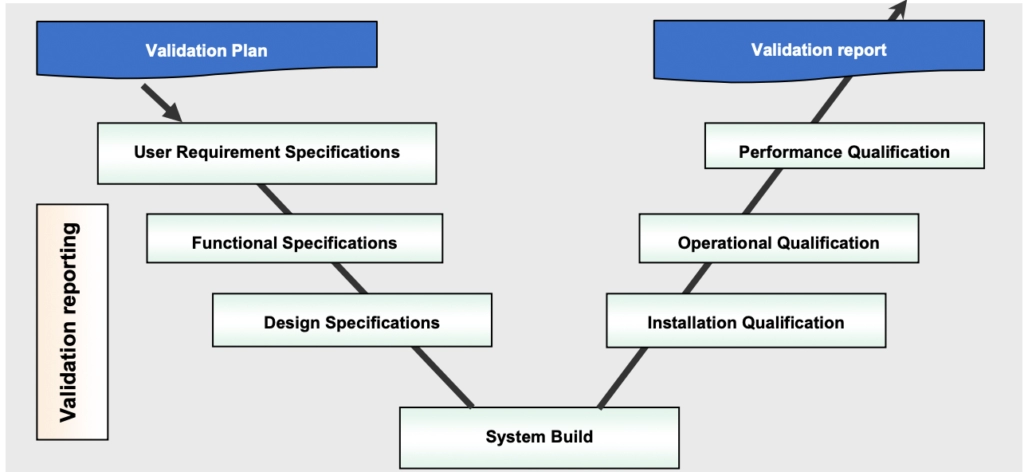
All The Validation Processes
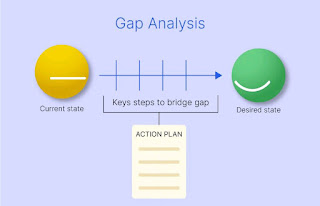
Gap Analysis
GAP here means incompleteness or missing links. GAP analysis means analyzing the SAP system through available documentation, and current status of SAP with reference to the guidelines of Regulatory agencies and attempt to find out from available implemented systems.
A gap analysis can be arranged on short notice. Depending on the scope and availability of documents and information, initial results are available after just six to eight weeks. A fixed price offer protects you against unwelcome surprises and ensures planning reliability.
Plan of Action
Based on our gap analysis we create action plan along with client, and decide which gaps can be closed technically and which are to be attended by other procedures. There may be some offset in closing the Gaps.
The pharmacy would be expected to tell us within 5 days of the action they intend to take to meet the standards and improve practice in the pharmacy. We will consider some flexibility in this timescale if there are exceptional reasons why this deadline cannot be met. At the 6-month inspection, the inspector will visit the pharmacy again to assess whether the pharmacy is meeting the standards and that the improvements are being sustained.

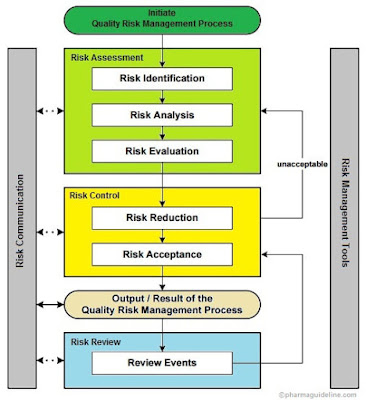
Risk Assessment
After the plan of action, we will carry out detailed Risk Assessment of relevant modules. If required the Human resource module and distribution part is also analyzed. In this step, we determine the important transaction codes and classify them into typical three categories as per GAMP guidelines.
The main objective of an SOP is to ensure that tasks are performed consistently, correctly, and to the required quality standards, which can lead to several benefits for pharmaceutical companies. Risk management principles are effectively utilized in many areas of business and government including finance, insurance, occupational safety, public health, pharmacovigilance, and by agencies regulating these industries. Although there are some examples of the use of quality risk management in the pharmaceutical industry today, they are limited and do not represent the full contributions that risk management has to offer.
Validation
This step is the formal start of validation process with the creation of VMP or Validation Master Plan. Then comes the protocols preparation. The validation scope, boundaries and responsibilities for each process or groups of similar processes or similar equipment's must be documented and approved in a validation plan.
Validation is the procedure which authorizing documentary evidences that prove, the following process/ method or activity will consistently produce the product which leads to the expected result (predetermined requirements).

