Cleaning Validation
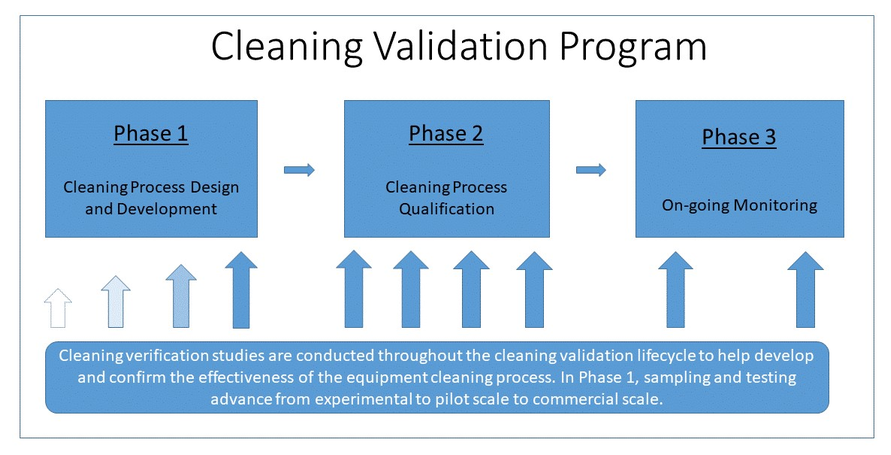
Cleaning validation establishes that the cleaning procedure can effectively remove product residue from pharmaceutical equipment to an acceptable limit. Cleaning validation verifies that the cleaning procedure can consistently and significantly reduce the amount of active ingredients, excipients, and cleaning agents to a concentration within the acceptance limit.
The practice of calling a piece of equipment clean just because it looks clean is not acceptable in the pharmaceutical manufacturing process.


Why is cleaning validation required?
During the manufacture of pharmaceutical products, the surface of the equipment comes in direct contact with therapeutic active ingredients and many excipients used in the formulation. Also, during cleaning and sanitizing, the equipment surface is treated with water and various cleaning agents.
Even a trace amount of these actives, excipients, or cleaning agents, if not removed properly during the cleaning process may cause harmful contamination to the next product. By doing so it ensures the purity, safety, and quality of the next product are not compromised.
What is a cleaning validation protocol?
A cleaning validation protocol can guide you through the entire validation process. Before starting any validation activity, the first thing you need is to devise a plan. Preparation of a cleaning validation protocol is the first step of planning.
Typically, in a protocol you will include a clear and specific objective. Followed by the collection or grouping of equipment that will be covered by the protocol. If you must adopt a protocol change, include the protocol change management approach.


What is FDA guidance on cleaning validation?
USFDA has published a guidance document “Guide to Inspections Validation of Cleaning Processes” where it has set some expectations for the firms to comply with when developing cleaning validation programs. To have written procedures (SOPs) detailing the cleaning processes used for various pieces of equipment.
To prepare specific written cleaning validation protocols in advance for the studies to be performed on each manufacturing system or piece of equipment which should address such issues as sampling procedures, and analytical methods to be used including the sensitivity of those methods.
Key considerations for cleaning validation
A cleaning validation project is a delicate operation. You have to follow a very specific methodology during cleaning validation works. While implementing a cleaning validation program, there are several key considerations you need to be aware of.
However, keep in mind that the types of equipment and their utilization in the manufacturing process have endless possibilities. Hence, one set of considerations may not fit for all circumstances.